Pharmaceuticals Company Secures Patent Approval For Lead Asset For Autoimmune Diseases
Conduit Pharmaceuticals Receives Patent Approval for Lead Asset Targeting Autoimmune Diseases.
Disclaimer: This article is intended for informational purposes only and does not constitute medical advice, endorsement, or recommendations. The contents are based on publicly available information as of July 17, 2024. Readers should consult with a healthcare professional for specific medical guidance.
Real-time information is available daily at https://stockregion.net
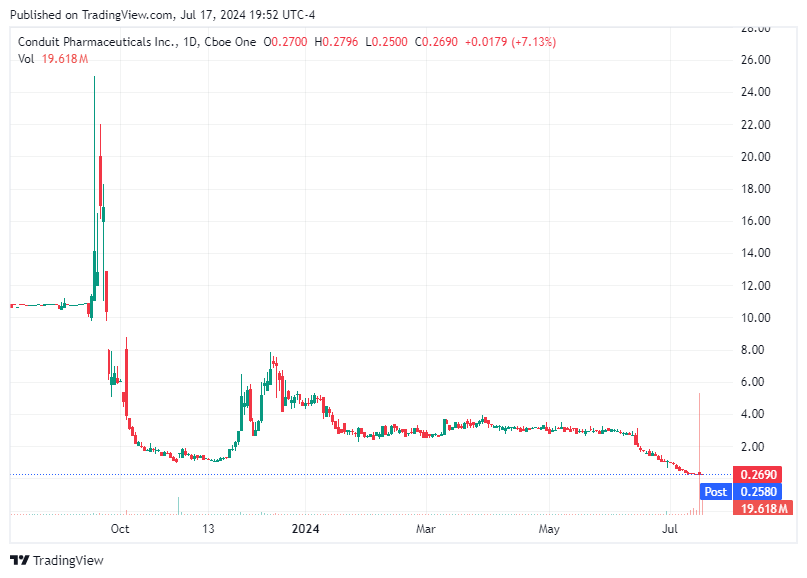
On July 17, 2024, Conduit Pharmaceuticals Inc. (Nasdaq: CDT) announced that it had received approval for a composition of matter patent application by IP Australia. This approval marks a milestone in the company's journey to develop its lead asset, a HK-4 Glucokinase Activator targeting a broad range of autoimmune diseases. This achievement has led to a notable increase in Conduit's stock price, up by 73% premarket at $0.4160.
Participation in the Patent Prosecution Highway (PPH)
The newly approved patent application, identified as 2022384750, pertains to the innovative solid-form technology employed by Conduit’s research and development team. Through this technology, the team successfully identified cocrystals of their lead asset, AZD1656, a HK-4 Glucokinase Activator. Cocrystals are unexpected formations that present unique opportunities in drug development, providing enhanced stability, bioavailability, and patent protection. Conduit Pharmaceuticals is enrolled in the Patent Prosecution Highway (PPH), an initiative designed to expedite the patent examination process in major jurisdictions including the United States, Europe, and Japan. Given the approval by IP Australia, Conduit anticipates a higher probability of obtaining subsequent approvals in these critical markets. Patents granted in these regions are highly coveted, offering up to 20 years of protection, which can enhance the marketability and commercial potential of drug products.
Cocrystals play a crucial role in the pharmaceutical industry, particularly in the context of drug substance patents. In the U.S. Food and Drug Administration’s (FDA) Orange Book, such patents are classified as “drug substance” patents. These patents not only bolster Conduit's intellectual property portfolio but also provide substantial leverage for future out-licensing opportunities. As highlighted by Jeffrey A. Lindeman, Ph.D., Conduit's consultant on cocrystal intellectual property, the creation of these cocrystals and the subsequent patent protection showcase the company's scientific acumen and foresight in drug product development.
Dr. David Tapolczay, Chief Executive Officer of Conduit Pharmaceuticals, emphasized the importance of the patent approval in Australia. He noted that the approval strengthens the company's intellectual property portfolio, securing up to 20 years of protection and classification as a drug substance. This validation of Conduit's R&D capabilities enhances their position for future out-licensing opportunities, thereby maximizing the potential value derived from their scientific innovations.
Business Model and Leadership
Conduit Pharmaceuticals operates a distinctive business model aimed at addressing unmet medical needs through cutting-edge solid-form technology. The company extends the intellectual property of its assets and collaborates with life science companies to commercialize these products.
Led by experienced pharmaceutical executives, Conduit Pharmaceuticals boasts a leadership team including Dr. David Tapolczay, who previously served as CEO of the UK-based medical research charity LifeArc, and Dr. Freda Lewis-Hall, former Chief Medical Officer of Pfizer, Inc., who chairs the Board. Their expertise and leadership are pivotal to driving the company’s initiatives and ensuring successful commercialization of their pipeline products. Conduit Pharmaceuticals' Phase II ready pipeline includes two key assets:
HK-4 Glucokinase Activator: This asset targets autoimmune diseases and has undergone over 20 clinical trials involving more than 1,000 patients. The recent patent approval further reinforces its potential impact in the treatment of autoimmune conditions.
MPO Inhibitor: This asset is believed to have the potential to treat idiopathic male infertility, representing another area of unmet medical need.
Autoimmune diseases represent a diverse group of conditions wherein the immune system mistakenly attacks the body’s own tissues. The development of therapies targeting these diseases is complex and requires a deep understanding of underlying mechanisms. The HK-4 Glucokinase Activator developed by Conduit showcases the company's commitment to pioneering treatments that offer novel solutions for these challenging conditions.
The approval of the composition of matter patent by IP Australia marks a pivotal achievement for Conduit Pharmaceuticals. This milestone not only validates the company's robust R&D efforts but also positions it favorably for future growth and out-licensing opportunities. As Conduit continues to advance its innovative pipeline, it holds promise for addressing critical unmet medical needs and enhancing patient outcomes through its cutting-edge technologies.
Disclaimer: The information provided in this article is for educational and informational purposes only and should not be construed as medical or legal advice. Readers should consult with a healthcare provider for any medical concerns or questions. This content is based on publicly available information and reflects the status as of the date of publication.
Real-time information is available daily at https://stockregion.net

