Stock Spikes In Volume: Therapeutics Company To Report First Quarter Results With Major Update
The first quarter results will provide investors with a snapshot of the company's financial health and operational efficiency.
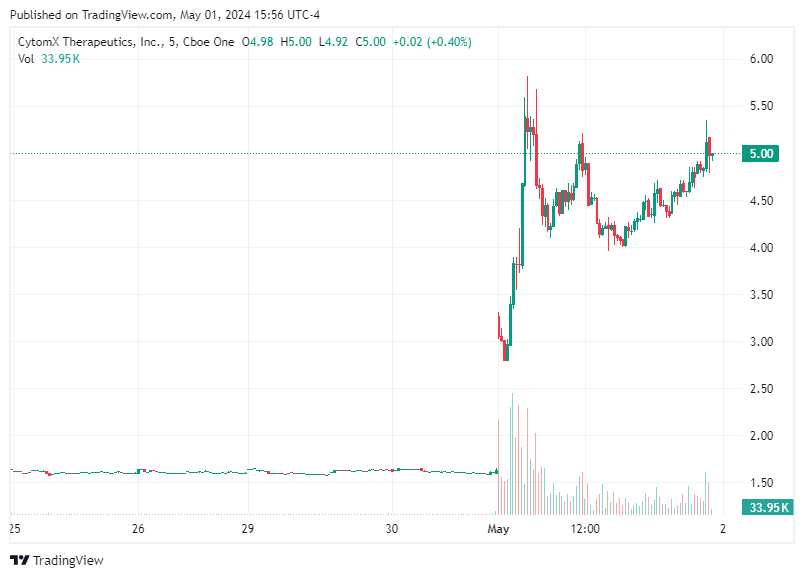
Innovation and precise targeting are key to advancing cancer treatment, CytomX Therapeutics has emerged as a pioneering force. The company's recent announcement regarding an increase in volume by 3.04 and testing of Volume Weighted Average Price (VWAP) levels signals a growing interest from the investment community, particularly ahead of its forthcoming update on the first quarter 2024 results and the initial CX-904 Phase 1a dose escalation study. This update, scheduled for Wednesday, May 8, 2024, after U.S. markets close, is eagerly anticipated by both investors and stakeholders within the oncology and biopharmaceutical fields.
CytomX is renowned for its clinical-stage endeavors focused on oncology, particularly its development of novel conditionally activated, masked biologics. These biologics are designed to localize to the tumor microenvironment, embodying a strategic approach to cancer treatment. Through its proprietary PROBODY® therapeutic platform, CytomX aims to revolutionize cancer therapy by enhancing the safety and efficacy of treatments. The company’s approach represents a significant departure from traditional methods, promising to deliver therapies that are more targeted and less toxic to patients.
The bedrock of CytomX’s innovative efforts is its PROBODY therapeutic platform. This platform illustrates the company’s commitment to creating safer, more effective therapies for cancer treatment. PROBODY therapeutics are designed to remain inactive until they are localized within the tumor microenvironment. This conditional activation minimizes the effects on healthy tissues, potentially reducing the side effects often associated with cancer therapies. CytomX's pipeline is a testament to its robust and differentiated approach, encompassing therapeutic candidates across various treatment modalities, including antibody-drug conjugates (ADCs), T-cell engagers, and immune modulators such as cytokines. Each of these candidates exemplifies the application of the PROBODY platform, targeting different aspects of cancer pathology with precision and specificity.
Among the noteworthy candidates in its clinical-stage pipeline are:
CX-904: A masked, conditionally activated T-cell-engaging bispecific antibody that targets the epidermal growth factor receptor (EGFR) on tumor cells and the CD3 receptor on T cells. In partnership with Amgen, CX-904 represents a collaborative effort in global co-development, highlighting its potential significance in the field.
CX-2051: A masked, conditionally activated ADC aimed at epithelial cell adhesion molecule (EpCAM), discovered in collaboration with Immunogen (now part of AbbVie). Its applicability across multiple EpCAM-expressing epithelial cancers underscores the versatile nature of CytomX's therapeutic strategy.
CX-801: A masked interferon alpha-2b PROBODY cytokine, demonstrating broad applicability in both traditionally immuno-oncology sensitive and insensitive (cold) tumors. This candidate exemplifies the potential of PROBODY therapeutics to address a wide range of tumor environments.
CytomX's strategic collaborations with industry leaders such as Amgen, Astellas, Bristol Myers Squibb, Regeneron, and Moderna reflect the company's prominent position within the oncology field. These partnerships not only underscore the credibility and potential of CytomX’s therapeutic candidates but also facilitate progress through shared expertise and resources. The upcoming conference call and webcast, set for May 8, will offer stakeholders an opportunity to gain insights into the company's first quarter 2024 results and the progress of CX-904's Phase 1a dose escalation study. The latter is of particular interest, given the potential of CX-904 to redefine the landscape of T-cell-engaging bispecific antibodies and its implications for patients with cancers expressing EGFR.
Implications for Investors and the Oncology Community
CytomX’s progress, especially in the context of CX-904, has significant implications for investors and the broader oncology community. For investors, the development of CX-904 and its inclusion in strategic collaborations points to potential growth and value generation, particularly as it advances through clinical trials. For the oncology community, the advancement of CX-904 and other candidates in CytomX's pipeline represents hope for more effective, less toxic cancer treatments. As CytomX Therapeutics prepares to unveil its first quarter 2024 results and provide an update on the CX-904 Phase 1a clinical data, the anticipation builds not only among investors but also among those closely following advancements in oncology therapeutics. The company's pioneering work in developing conditionally activated, masked biologics, powered by its PROBODY therapeutic platform, stands at the forefront of innovation in cancer treatment. With a focus on creating safer, more effective therapies, CytomX continues to pave new paths in the quest to conquer cancer, promising a future where cancer treatments are not only effective but also inherently considerate of patient well-being.
The announcement by CytomX Therapeutics of its impending first quarter 2024 results, coupled with an update on the initial CX-904 Phase 1a dose escalation study, is poised to have a significant impact on both the company and its stock ($CTMX) in the immediate term. Several factors come into play when assessing how this news might influence investor sentiment, stock volatility, and the company's market valuation.
Anticipation Ahead of Results
The increase in volume by 3.04 and testing of VWAP (Volume Weighted Average Price) levels ahead of the announcement indicates heightened trading activity, which often signifies increased interest from investors and traders alike. This anticipatory movement suggests that the market is closely watching CytomX, with investors potentially positioning themselves to react to the upcoming news. Positive results and promising updates regarding CX-904 could amplify this interest, driving up the stock's demand and, subsequently, its price. The first quarter results will provide investors with a snapshot of the company's financial health and operational efficiency. Key metrics such as revenue growth, research and development expenses, and cash burn rates will be scrutinized closely. For a biopharmaceutical company like CytomX, which is in the clinical stage, financial stability is critical for sustaining ongoing and future research projects. Strong financial results could bolster investor confidence, leading to a positive impact on the stock's performance.
Perhaps more pivotal than the financial results is the update on the CX-904 Phase 1a dose escalation study. CX-904, being a part of CytomX's pipeline focusing on novel cancer therapeutics, represents a significant component of the company's potential value proposition to investors. The outcome of this study and the developmental progress of CX-904 are crucial, as they directly impact CytomX's future prospects and its ability to attract further investment or partnership opportunities. Positive preliminary data demonstrating safety, tolerability, and efficacy could significantly uplift the stock, as it would validate the company's PROBODY® therapeutic platform and its application in oncology. The combined effect of the financial results and the CX-904 update will likely influence investor sentiment substantially. Positive news can lead to bullish behavior, driving up the stock price, while any setbacks or delays could result in bearish trends. It's essential to note that the biopharmaceutical sector is particularly sensitive to clinical trial outcomes and regulatory milestones, which can cause significant stock volatility.
Long-term Implications
While the immediate impact of the announcement on May 8, 2024, is critical, the long-term implications should not be overlooked. Success in early-phase trials like the CX-904 Phase 1a dose escalation study can set the stage for subsequent phases of clinical development, potentially leading to accelerated pathways, strategic partnerships, and increased funding opportunities. Conversely, unfavorable results could necessitate a reassessment of strategy, impacting the company's long-term prospects and stock trajectory. CytomX Therapeutics' upcoming announcement holds the potential to markedly influence its stock in the short term, with the specifics of the first quarter results and the CX-904 update acting as key determinants. Investors and market analysts will be keenly observing these developments, as they could signal the future direction of $CTMX. Regardless of the outcome, this period promises to be a pivotal moment for CytomX, its stakeholders, and its commitment to advancing cancer treatment through innovative therapeutics.


